반응 용어 정리
- substrate: 기질 = starting material
- reagent: 반응물
- product: 생성물
- byproduct: 부산물 (desired)
- side product: 다른 반응으로 간 생성물 (undesired)
Nucleophilic substitution reaction(SN reaction)
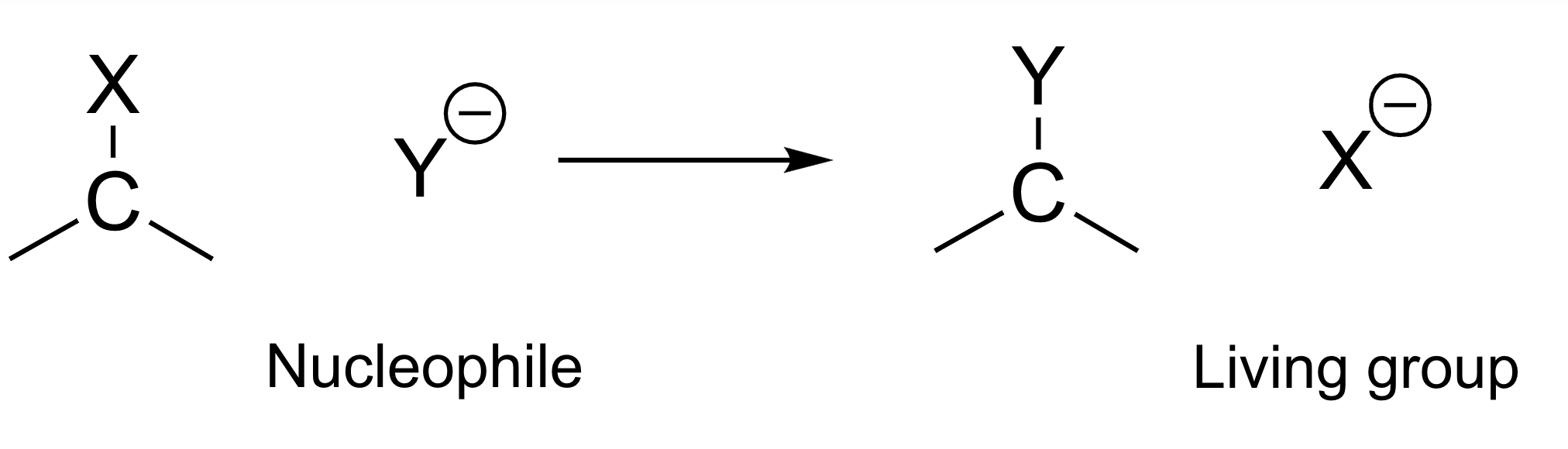
- nucleophile (Nu-) : 양성자 선호, or ()
- electrophile (E-) : or ()
-
Alkyl halide
- halogen: 전기음성도 좋은 electrophile
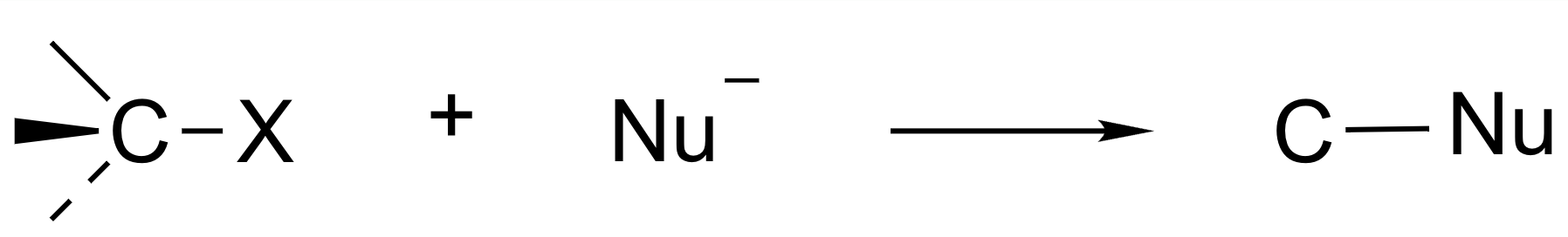
- halogen: 전기음성도 좋은 electrophile
-
synthesis from alkyl halides through SN reactions

- possible mechanisms for substitution reactions
1) concerted (simultaneous) mechanism
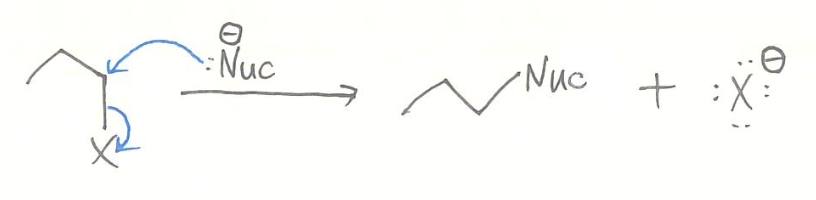
2) stepwise mechanism
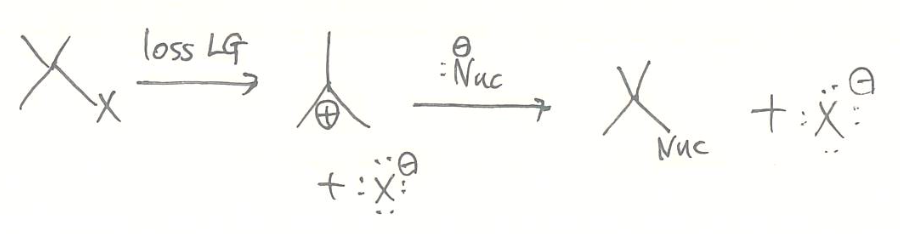
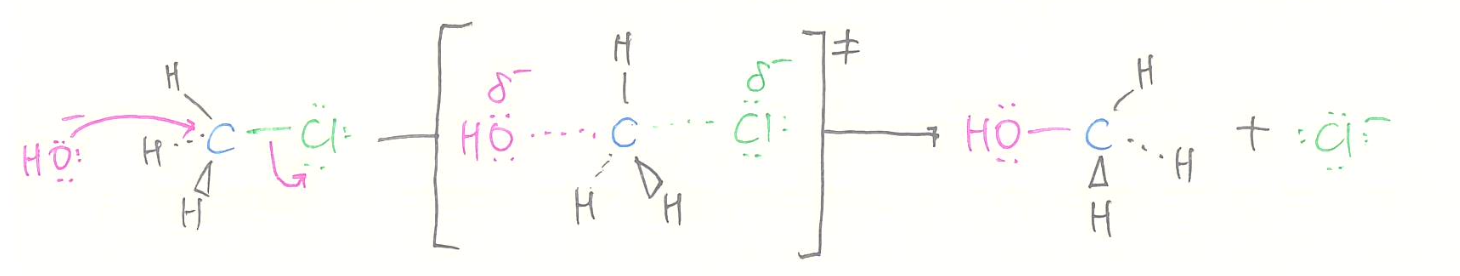
SN2
- kinetics
- rate = [alkyl halide][nucleophile]
- bimolecular process
- stereochemistry
- 100% inversion of configuration (back-side attack)
- stereospecific reaction
- factors determining a reactivity of SN2 reaction
- Leaving-Group's ability
- acidity of H-X
짝산 강산이면 짝염기 good LG
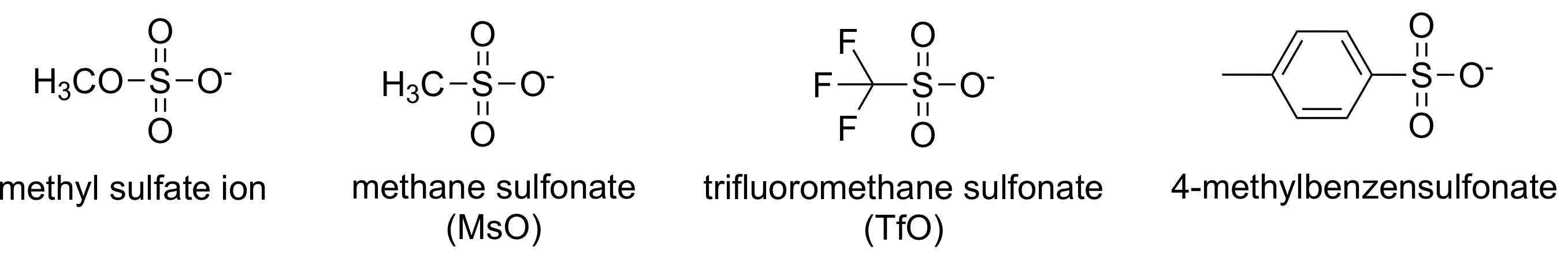
- sulfonates
- steric of in electrophile
- reactivity: methyl > 1 > 2 >> 3 (3: reaction (X))- nucleophilicity of nucleophile
- same period: basicity () nucleophilicity ()
- same group: polarizability () nucleophilicty () (HSe > HS > HO, PH > NH)- solvent effect
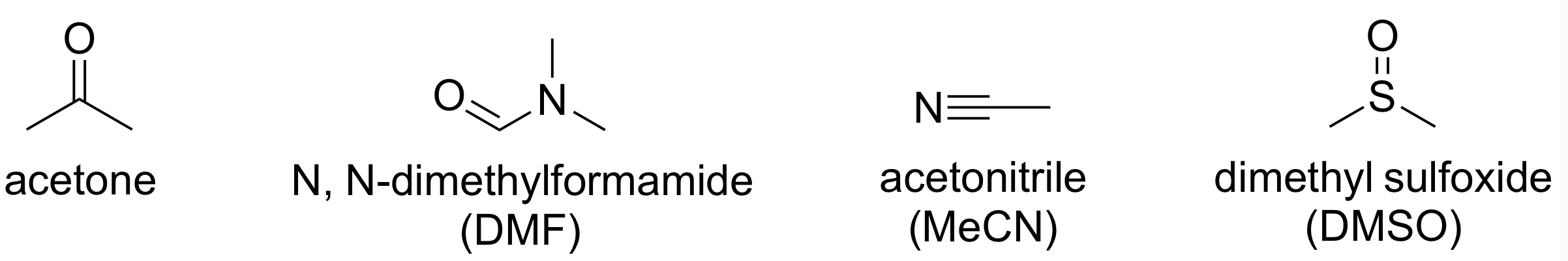
- polar protic < polar aprotic
protic solvent가 X 안정화
-
Strong Nu Weak Nu I Br Cl HS HO NC HO ROH RS RO -
aprotic solvents
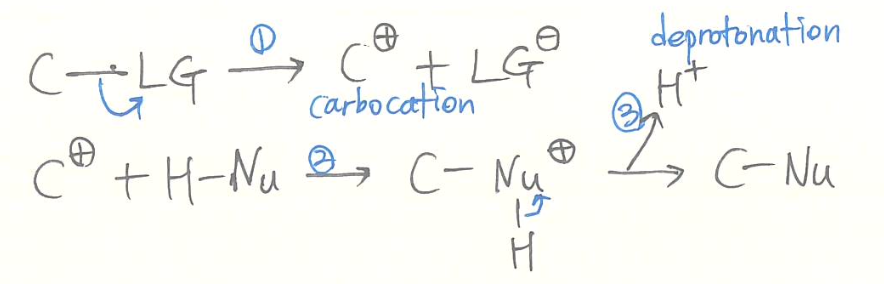
SN1
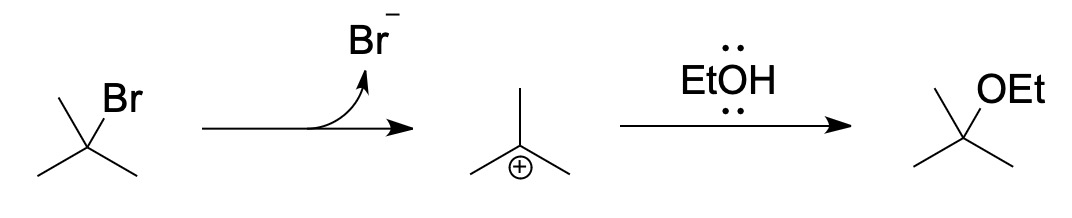
- kinetics
- rate = [substrate]
- unimolecular reaction
- reaction mechanism (1st step: rds)
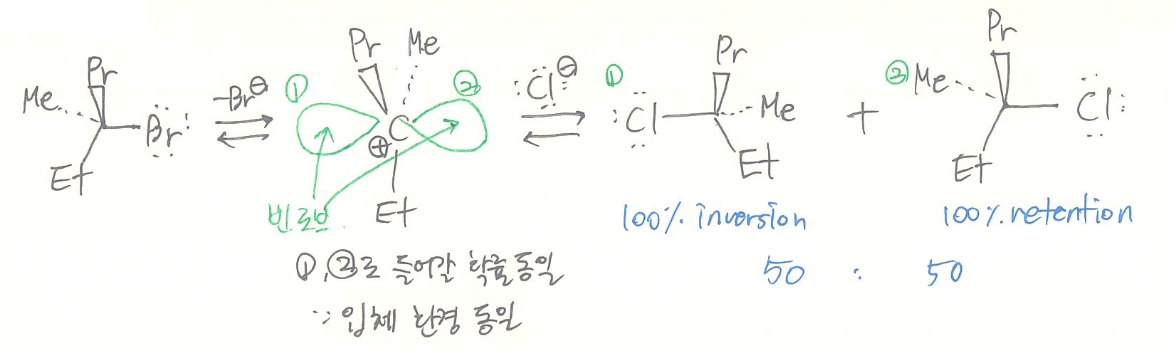
- stereochemistry
- racemization
- reactivity
- LG's ability
- better LG faster SN1- nucleophilicity: no effect
- steric of electrophile
- : 3 >> 2 > 1
HYPERCONJUGATION- solvent effect
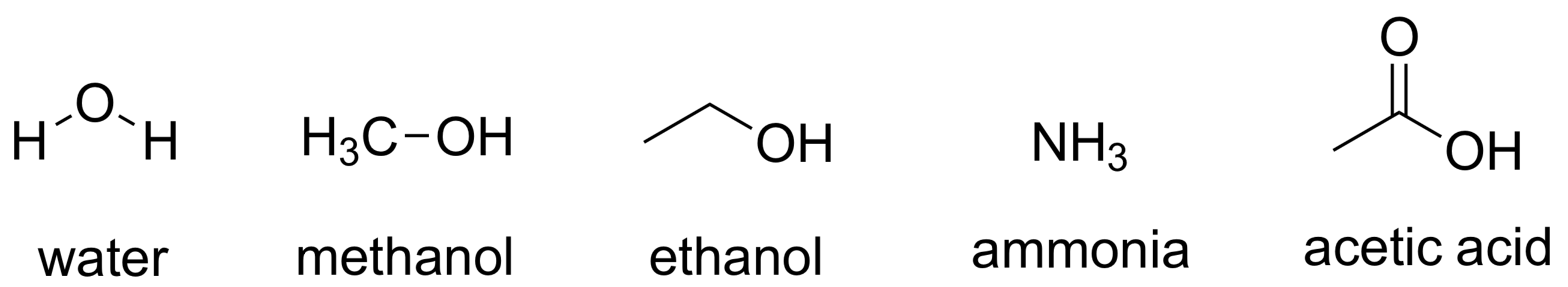
- polar aprotic < polar protic
H-Nu & C-LG 전기적 중성 protic solvent 써도 안정화 (X)- additional process
- proton transfer: poor LG 경우, H 넣어서 better LG로.
- carbocation rearrangement
- protic solvents
[ SN2 vs. SN1 ]
- Nucleophile
- Nu가 charge (O) SN2
- Nu가 charge (X), 용매겸 Nu SN1
- Solvent effect
- polar aprotic: SN2
- polar protic: SN1
Reference
- Klein, D. R. (2021). Organic Chemistry. John Wiley & Sons.
- 2023-1 DGIST 유기화학1 수업자료
- The structures in this document were drawn by ChemDraw JS.

좋은 정보 감사합니다